您可以通过点击 CST 信号转导通路图的各个节点查找研究资源或产品信息。您还可以下载通路图,将其用于教育和研究。
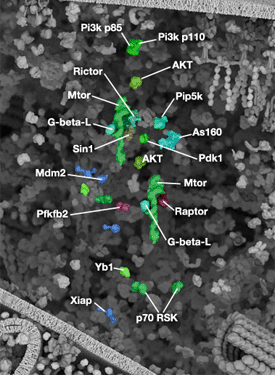
丝氨酸/苏氨酸激酶 Akt/PKB 是存在于哺乳动物中的三种同工型。Akt1 具有广泛的组织分布,Akt2 主要分布在肌肉和脂肪细胞中,而 Akt3 在睾丸和大脑中表达。Akt 调控多个生物过程,包括细胞存活、增殖、生长和糖原代谢。许多生长因子、激素与细胞因子通过结合其同源受体酪氨酸激酶 (RTK)、细胞因子受体或 GPCR,以及通过触发脂质激酶 PI3K 的活化来激活 Akt,从而在细胞质膜中生成 PIP3。Akt 通过其普列克底物蛋白同源 (PH) 结构域与 PIP3 相结合,导致 Akt 转位至细胞膜。通过双磷酸化机制,可以激活 Akt。同样因为自身 PH 结构域而转位至细胞膜的 PDK1,能够在活化环中的 Thr308 位点使 Akt 磷酸化。羧基末端 Ser473 位点的二次磷酸化对于活性来说也是必要的,并由 mTOR-rictor 复合体 mTORC2 完成。
PTEN,一种催化 PIP3 去磷酸化的脂质磷酸酶,是 Akt 信号转导的主要负调节分子。PTEN 功能的丧失与许多人类癌症存在关联。磷酸酶 PP2A 与 PHLPP、化学调节物 wortmannin 与 LY294002 也都是 Akt 活性的负调节因子,后两者均是 PI3K 的抑制剂。
被激活的 Akt 会使大量下游底物磷酸化,包括共有序列 RXRXXS/T。其主要功能之一是通过调控 mTOR 信号转导通路促进细胞生长与蛋白质合成。Akt 直接使 mTOR 磷酸化且将其激活,并抑制 mTOR 抑制剂蛋白 PRAS40 与马铃薯球蛋白 (TSC2)。通过 p70 S6 激酶的信号转导和抑制 4E-BP1,这些作用共同促进细胞生长、G1 细胞周期进程。
GSK-3 是 Akt 的主要靶标,而 GSK-3α (Ser21) 或 GSK-3β (Ser9) 的抑制性效应的磷酸化具有许多细胞效应,例如:促进糖原代谢、细胞周期进程、调控 wnt 信号转导以及形成阿尔茨海默病中的神经原纤维缠结。Akt 通过使数个促凋亡靶标(包括 Bad、Bim、Bax 以及叉头框 (FoxO1/3a) 转录因子)磷酸化和失去活性,从而直接促进细胞存活。Akt 也在代谢与胰岛素信号转导中发挥重要作用。通过 Akt 的胰岛素受体信号转导能够通过激活 AS160 和 TBC1D1,促进 Glut4 转位,从而导致葡萄糖摄取增加。Akt 通过对 PFK 和己糖激酶进行磷酸化来调控糖酵解,并在癌细胞的无氧糖酵解(也称为 Warburg 效应)中发挥重要作用。
异常 Akt 信号转导是某些病理中的潜在缺陷。Akt 是人类癌症中最常被激活的激酶之一,因为具有持续活性的 Akt 可以促进未经调控的细胞增殖。Akt2 信号转导异常会因为葡萄糖稳态上的缺陷而引发糖尿病。Akt 还因其在心脏生长、血管生成和心脏肥大中的作用,成为心血管疾病中的重要参与因素。